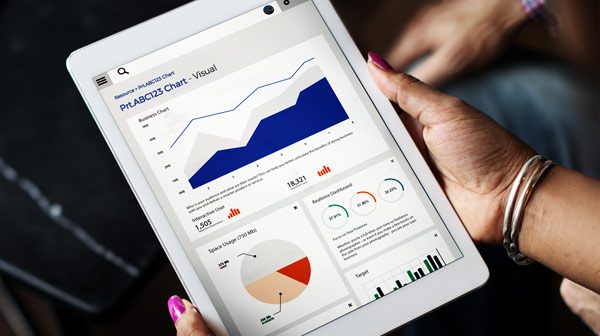
Clinical trials create a relentless stream of unstructured data. Expert clinical data management, security and user access are all factors that need to be carefully planned, or your experimental, logistical and patient data quickly becomes an unforgiving beast capable of slowing down business decisions. Technology can of course tame it – if you’re up for the task. So what does it take to tuck multiple workflows behind a single accessible interface? We have tackled it and lived to tell the tale….
Effective data management
In certain environments such as manufacturing, logistics, distribution and returns, the volume of data is always extensive and usually pours in from different (often incompatible) sources. How do you get a picture of what’s happening all the way from manufacturing to market when multiple providers are operating their own proprietary systems? The only answer is to integrate them.
Start by assembling a team of project leads and subject matter experts from key disciplines around the organisation. Get together to agree on the scope of the project and the resources required. Work through the customer journeys, step by step, marking where data is collected, transformed, shared and what gaps, limits or inaccuracies may exist. This type of data-led approach allows the team to ‘walk the process’ figuring out where a portal could make the greatest impact, create the most value and assist the widest selection of teams.
Reporting and visualisation made easy
In a recent project we discovered poor reporting options were a hindrance. The available reports offered too much data, not enough filters or were poorly presented. Previous systems had allowed limitless ad hoc reports to be generated. There were literally thousands of unused reports, hundreds of identical copies and many of these reports were being emailed daily to people who had left their role, or the business. It was a maintenance nightmare.
The project team wanted to retain flexibility but maintain order. A flexible template was adopted allowing 80% of reporting requests to be addressed by a smaller set of templates. By designing the output first the team were able to work backwards designing-in changes to the transformation and collection stages that would transform the quality of the output. Scientists love data and data visualisations that allow science to be done better or faster.
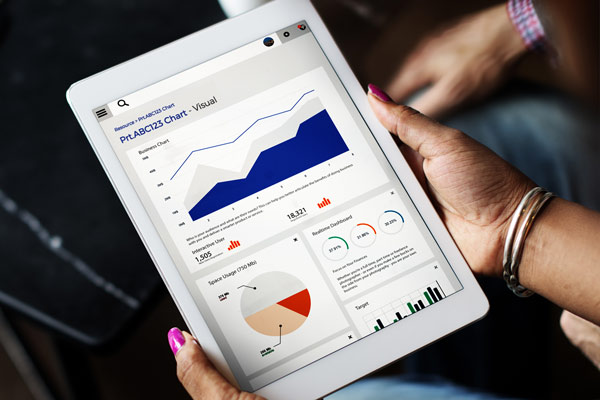
The design effort focused on presenting granular data with intuitive filters, aggregations, trends and calculations in a highly visual form. With user experience firmly at the heart of the design process the aim was achieved – to make business decisions based on actionable insight easier. (Invest in this. Extend that. Ship these.)
Managing multiple workflows through a single interface and collaborating with remote colleagues through a shared platform yielded faster analysis, easier dissemination of information and better decision making. All in all, a better use of time for everyone involved.
—–
Want to consult a clinical trials specialist? Hire a freelance expert on Kolabtree.